Write the Complete Electron Configuration for the Iron Atom
Of e- Element atom e-. This problem has been solved.
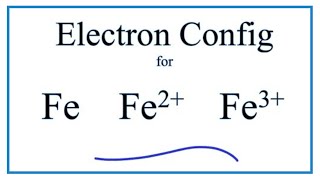
Electron Configuration For Iron Fe Fe2 And Fe3
Write the complete electron configuration for the neon atom.
. Using NOBLE GAS notation write the electron configuration for the carbon atom. Using NOBLE GAS notation write the electron configuration for the copper atom. The iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ionFe 3.
How Many Outer Electrons Does It Have-The electrons which are present in the outermost valence shell of an atom are called the valence electrons of the atom. When we write the configuration well put all 20 electrons in orbitals around the nucleus of the Calcium atom. Since the 3s if now full well move to the 3p where well place the remaining six electrons.
How will the electron configuration of the atom change when the atom becomes an iron. Its valence orbitals are the 4s and 3d s. The iron Fe atom is located in the d-block of the periodic table specifically in the 3d and it has 26 electrons in the structure.
Writing the electron configuration you really only need the valence orbitals and you can omit the core orbitals by notating it via. Consider The Electron Configuration For Iron. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
Both of the configurations have the correct numbers of electrons in each orbital it is just a matter of how the electronic configuration notation is written here is an explanation why. -From the electronic configuration of the iron atom we come to know that the number of elect. A Write the complete electron configuration for the iron atom B Using noble gas notation write the electron configuration for the maganese atom.
Write the complete electron configuration for the chlorine atom. In writing the electron configuration for Calcium the first two electrons will go in the 1s. Note that when writing the electron configuration for an atom like Cu the 3d is usually written before the 4s.
The next six electrons will go in the 2p orbital. Write the complete electron configuration for the zinc atom. Nickel Nitrogen Zinc Potassium Write the symbols for the following elements.
Si 14 electrons b. Write the complete electron configuration for the nickel atom. Note that when writing the electron configuration for an atom like Fe the 3d is usually written before the 4s.
What we have is. Write the complete electron configuration for the oxygen atom. In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom there are 20 electrons.
Iron is on the fourth row of the periodic table sixth column of the transition metals atomic number 26. The complete electron configuration of iron contains 26 electrons because 2 2 6 2 6 6 2 122 6 4 s 2. Write the complete electron configuration for the iron atom.
Of the elements discussed in the video iron is most like Sc but iron has 5 more electrons in the last subshell which is filled with electrons making its configuration end in. Copper platinum calcium manganese iron barium lead strontium Write the symbols for the following elements. Both are explained in the video below.
Ground state electron configurations. Write the complete electron configuration for the nickel atom. Using NOBLE GAS notation write the electron configuration for the zinc atom.
Using NOBLE GAS notation write the electron configuration for the carbon atom. The p orbital can hold up to six electrons. Write the complete.
Using NOBLE GAS notation write the. Using NOBLE GAS notation write the electron configuration for the chromium atom. A Write the complete electron configuration for the iron atom B Using noble gas notation write the electron configuration for the maganese atom.
Using NOBLE GAS notation write the electron configuration for the carbon atom. Memorize flashcards and build a practice test to quiz yourself before your exam. Write the complete electron configuration for each atom.
Using NOBLE GAS notation write the electron configuration for the chromium atom. Write the complete electron configuration for the neon atom. Using NOBLE GAS notation write the electron configuration for the calcium atom.
The correct and complete electron configuration is. Zincs complete electron configuration is 1s22s22p63s23p64s23d10. 1s2 2s2 2p6 3s2 3p6 4s2 3d6 To write an electron configuration for any element youll need to apply the Aufbau principle and use a diagonal diagram.
Si 14 electrons Write the complete electron configuration for each atom. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Its core orbitals are the 1s 2s 2p s 3s and 3p s.
Answered by Eliza Jones on Tue Sep 7 2021 1221 AM. Using SPECTROSCOPIC notation write the complete electron configuration for the iron atom. The atomic number of iron is 26.
Start studying the Chemistry Chapters 4 6 flashcards containing study terms like Name the Elements symbol. Up to 256 cash back Using NOBLE GAS notation write the electron configuration for the vanadium atom. Well put six in the 2p orbital and then put the next two electrons in the 3s.
Using NOBLE GAS notation write the electron configuration for the magnesium atom. Therefore the Argon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6. In order to write the electron configuration for Iron Fe we first need to know the number of electrons for the Fe atom there are 26 electrons.
Fe 3e Fe 3 The electron configuration of iron ionFe 3. Using NOBLE GAS notation write the electron configuration for the iron atom. Write the complete electron configuration for the oxygen atom.

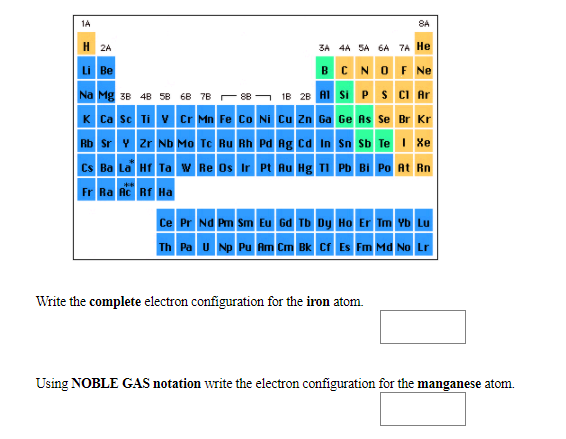
Solved 8a H 2a 3a 4a Sa 6a Ta He Li Be Write The Complete Chegg Com
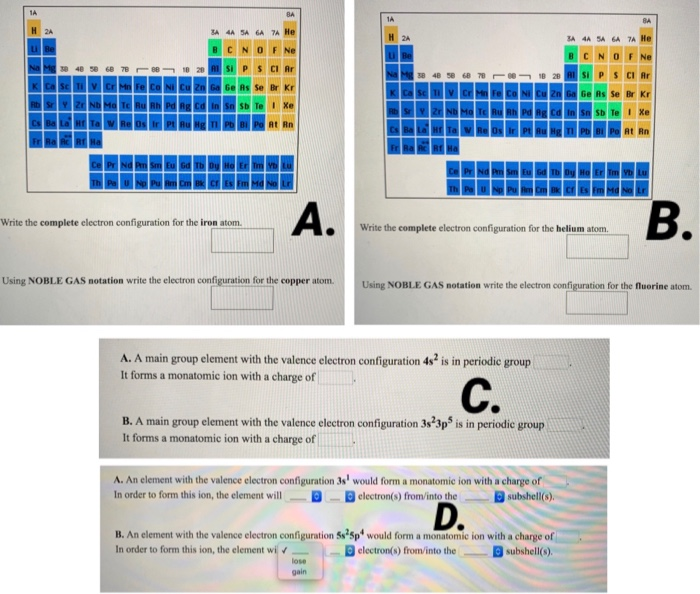
Solved H 2a B Write The Complete Electron Configuration For Chegg Com

0 Response to "Write the Complete Electron Configuration for the Iron Atom"
Post a Comment